'김갑성' 기자의 전체기사
-

Clarivate Identifies Eleven Potential Blockbuster and Transfo…
Anticipated advancements in weight loss, diabetes, oncology, rare conditions and protein degraders poised to reshape patient care LONDON, Jan. 6, 2026 -- Clarivate Plc (NYSE: CLVT), a leading global provider of transformative intelligence, today released its 2026 Drugs to Watch report, identifying eleven therapies expected to deliver significant clinical impact and strong commercial potential over the coming year. The annual analysis highlights therapies expected to reshape treatment approaches across metabolic disease, oncology, immunology, rare conditions and neurological dis
- 김갑성 기자
- 2026-01-06 16:21
-
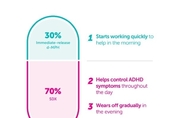
Ark Biopharmaceutical Receives China Marketing Authorizatio…
SHANGHAI, Jan. 6, 2026 -- Shanghai Ark Biopharmaceutical Co., Ltd. ("ArkBio") today announced that China's National Medical Products Administration (NMPA) has approved the New Drug Application for Serdexmethylphenidate Chloride and Dexmethylphenidate Hydrochloride Capsules (Azstarys®) for treatment of Attention Deficit Hyperactivity Disorder (ADHD) in patients 6 years of age and older in China. Azstarys® is a central nervous system stimulant indicated for the treatment of ADHD. Each once-daily capsule contains serdexmethylphenidate (SDX), a prodrug of dexmethy
- 김갑성 기자
- 2026-01-06 14:45
-
Esco Aster Signs Exosome Clinical cGMP Manufacturing Con…
SINGAPORE, Jan. 6, 2026 -- Esco Aster, a vertically integrated cell and derivatives CRDMO based at JTC LaunchPad Singapore, announced CMC manufacturing support for Shine-On Biomedical's HLA-G targeted exosome program. Shine-On Biomedical sponsored Esco Aster in 2023 for cGMP services, starting with high-yield exosome development using Esco Aster's cell line platform. The technical reports of process, analytical, and formulation development, exosome drug loading, GMP engineering runs, and stability studies supported Shine-On's IND submission. The IND was cleared by the U.S. FDA in Q1 2025. &
- 김갑성 기자
- 2026-01-06 12:03
-
Everest Medicines to Present at the 44th Annual J.P. Morgan H…
SHANGHAI, Jan. 6, 2026 -- Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the discovery, clinical development, manufacturing, and commercialization of innovative therapeutics, today announced that Ian Woo, President and Chief Financial Officer, will present a company update and that the management team including chairman Bill Wu and Chief Executive Officer Rogers Luo will participate in one-on-one meetings at the 44th Annual J.P. Morgan Healthcare Conference being held January 12-15, 2026, in San Francisco: 44th
- 김갑성 기자
- 2026-01-06 07:58
-

Kelun-Biotech Receives Investigational New Drug Approval fo…
CHENGDU, China, Jan. 5, 2026 -- Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. ("Kelun-Biotech" or the "Company", 6990.HK) announced that its Investigational New Drug (IND) application for SKB105 (also known as CR-003), an internally developed integrin beta-6 (ITGB6)-targeted antibody-drug conjugate (ADC), has been approved by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) of China for the treatment of advanced solid tumors. In December 2025, Kelun-Biotech and Crescent Biopharma, Inc. ("Cresc
- 김갑성 기자
- 2026-01-05 20:00
-
Sisram Medical Reports Completion of NIFDC Testing for DAXXIFY…
[ 메디채널 김갑성 기자 ] Marking a Key Milestone Toward Market Launch and Strengthening Sisram's Premium Aesthetics Portfolio HONG KONG, Jan. 5, 2026 -- Sisram Medical Ltd (the "Company" or "Sisram", 1696.HK; together with its subsidiaries collectively referred to as the "Group"), a global wellness group offering Energy-Based Devices (EBD), injectables, diagonostics, and other complementary solutions, today announced that its long-acting botulinum toxin type A product, DAXXIFY (trademark in Chinese Mainland达希斐®), has passed the quality testing from National I
- 김갑성 기자
- 2026-01-05 17:36
-

Kelun-Biotech Announces Breakthrough Therapy Designa…
CHENGDU, China, Jan. 5, 2026 -- Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. ("Kelun-Biotech" or the "Company," 6990.HK) today announced that its TROP2-directed antibody-drug conjugate (ADC) sacituzumab tirumotecan (sac-TMT, also known as SKB264/MK-2870) (佳泰莱®) in combination with MSD's anti-PD-1 monoclonal antibody pembrolizumab (KEYTRUDA®[1]) was granted Breakthrough Therapy Designation (BTD) by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) of China for the first-line treatment of patients with locally ad
- 김갑성 기자
- 2026-01-05 17:29
-

GenEditBio Receives FDA Clearance of IND Application for Its Le…
HONG KONG, Jan. 5, 2026 -- GenEditBio Limited ("GenEditBio"), a clinical-stage biotechnology startup focusing on genome-editing therapeutic solutions, today announced that the U.S. Food and Drug Administration (FDA) has cleared the company's Investigational New Drug (IND) application to initiate Phase 1/2 CLARITY trial activities for its lead in vivo genome-editing program GEB-101 for TGFBI corneal dystrophy. The Phase 1/2 CLARITY trial will collect initial data on the safety, tolerability and efficacy of GEB-101 in corneal dystrophy patients with TGFBI
- 김갑성 기자
- 2026-01-05 12:07
-
C&R Research, the 'K-Clinical Hub', Strengthens Partnerships…
[ 메디채널 김갑성 기자 ] Accelerating Indonesia Collaboration through Global Clinical Capabilities SEOUL, South Korea, Jan. 5, 2026 -- C&R Research, founded in 1997 as Korea's first full-service Contract Research Organization (CRO), has played a pivotal role in advancing the Korean clinical trial industry and supporting the global expansion of Korean clinical research. With expertise across next-generation modalities, C&R Research has established itself as a key partner in global drug development. Leveraging its global subsidiaries and international network, C&R Research is
- 김갑성 기자
- 2026-01-05 10:00
-
Ascletis Announces U.S. FDA IND Clearance for 13-Week Phase…
-The Phase II study for diabetes is a 13-week, randomized, double-blind, placebo-controlled and multi-center study to evaluate the efficacy, safety, and tolerability of ASC30 in participants with diabetes. Enrollment is expected to begin in the first quarter of 2026.-ASC30 demonstrated placebo-adjusted weight loss of 7.7% in a recently completed 13-week U.S. Phase II study in participants with obesity or overweight, with better gastrointestinal tolerability. No hepatic safety signal was observed. HONG KONG, Jan. 5, 2026 -- Ascletis Pharma Inc. (HKEX: 1672, "Ascletis") announc
- 김갑성 기자
- 2026-01-05 08:10
- 1포리프 굿마팻 (누바디 굿바이 마이팻) 다이어트 건강기능식품 출시
- 22021년 식품분석전문가 1급/2급 검정자격시험 안내
- 3투썸플레이스, 초콜릿 애프리콧 무스 출시
- 4아임웹, CRM 기능 출시… 고객 행동 데이터 기반 마케팅 지원
- 5하만카돈, AURA STUDIO 4 블루투스 스피커 출시
- 6대구오페라하우스 - 20주년 기념 골든 보이스 시리즈Ⅲ, 테너 콘서트
- 7만성질환자들의 유료독감백신접종에 정책적인 배려가 필요하다
- 82021년 대학생기자단 모집
- 9대한약물영양의학회 2020년 추계학술대회
- 10대한영양제처방학회 춘계학술대회 성황리에 마쳐
2026-01-31_SAT
- Sanyou Bio's Decade: From an Intelligent Super-Trillion Antibody Library to the "Innovation Hub for Original Drug Discovery" 16:15
- CANADA BACKS NEW DEFENCE, SECURITY AND RESILIENCE BANK 12:05
- Origin Agritech Announces Fiscal Year 2025 Results 06:00
- White Pearl Acquisition Corp. Announces Pricing of $100 Million Initial Public Offering 05:47
- Planet Green Holdings Corp. Provides Response to Unusual Market Action 05:05
- SKF completes previously announced divestment of non-core aerospace operation in Elgin, USA 03:36
- DOCOMO Concludes Partnership Agreement with Aduna to Advance Global Network API Expansion 03:34






