'김갑성' 기자의 전체기사
-

International Symposium on Myofunctional Therapy Conclu…
SHANGHAI, Nov. 5, 2025 -- The International Symposium on Myofunctional Therapy in Multidisciplinary Collaboration, hosted by the Chinese Association of Orofacial Myology (CHAOM) and supported by Smartee Denti-Technology, concluded successfully in Shanghai. The event brought together leading clinicians, educators, and researchers from China and the United States to examine the evolving role of orofacial myofunctional therapy (OMT) in contemporary orthodontic practice. OMT Gains Ground as a Critical Component of Orthodontic Diagnostics and Treatment Planning Keynote speake
- 김갑성 기자
- 2025-11-06 15:35
-
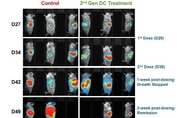
Lunai Bioworks achieves complete regression of both prim…
Lunai Bioworks Announces Publication of Second-Generation Allogeneic Dendritic Cell Therapy Platform in the journal "Vaccines". LOS ANGELES, Nov. 6, 2025 -- Lunai Bioworks (NASDAQ: LNAI), an AI-powered drug discovery and biodefense company, has announced a major scientific breakthrough: its next-generation immune cell therapy led to complete regression of both primary and metastatic pancreatic tumors in humanized mouse models. The study was published in a new Brief Report in Vaccines on November 2, 2025. The peer-reviewed Brief Report details the develo
- 김갑성 기자
- 2025-11-06 14:11
-

THE ADECCO GROUP Q3 2025 RESULTS
AD HOC ANNOUNCEMENT pursuant to Art. 53 Listing Rules of SIX Swiss Exchange Strong share gains, good growth and improved profit margin ZURICH, Nov. 6, 2025 -- HIGHLIGHTS Further strong market share gains, Group +375 bps and Adecco +300 bps Group revenues +3.4% yoy, and +3.0% qoq, with all GBUs improving sequentially Adecco GBU revenues +4.5% yoy; Europe returned to growth; Americas +20% yoy, APAC +9% yoy Akkodis GBU revenues -3% yoy; German turnaround progressing well LHH GBU revenues +4% yoy, led by CT +9% yoy, Ezra +59% yoy Healthy 1
- 김갑성 기자
- 2025-11-06 13:45
-
Alphamab Oncology Showcases Cutting-edge Breakthroug…
SUZHOU, China, Nov. 6, 2025 -- The 16th World ADC San Diego, held from November 3–6, 2025, brought together industry leaders in San Diego, USA. Dr. Ting Xu, Founder, Chairman, and CEO of Alphamab Oncology, was invited to deliver a presentation titled "JSKN021: EGFR/HER3 Targeted Dual-payload ADC", showcasing the company's cutting-edge technological breakthroughs and innovative achievements in the ADC field to a global audience. The modularly designed dual-payload ADC platform technology and the prominent preclinical efficacy of JSKN021 garnered attention from attendees, making it on
- 김갑성 기자
- 2025-11-06 12:05
-
The 2025 Chinese Congress on Holistic Integrative Oncolog…
KUNMING, China, Nov. 6, 2025 -- From November 6 to 9, 2025, the highly anticipated 2025 CCHIO will be grandly held in Kunming, the "Spring City". Hosted by the Chinese Anti-Cancer Association and the Secretariat of the Tengchong Scientists Forum Organizing Committee, co-hosted by the World Association of Integrative Oncology (WAIO) and the China Institute of Integrative Medicine Development Strategy, and jointly organized by Yunnan Cancer Hospital, Kunming Medical University and Yunnan Anti-Cancer Association, this congress aims to become a significant milestone for academic exchang
- 김갑성 기자
- 2025-11-06 10:58
-

New Regional Certifications to Boost Healthcare Training Stan…
Two new specially designed certification programmes will train doctors, nurses and technical specialists across the region in simulation-based medical education. SINGAPORE, Nov. 6, 2025 -- The first healthcare simulation certification programmes designed specifically for Southeast Asian professionals were launched at the S3 Conference 2025 opening ceremony today. The event was attended by Mr Tan Kiat How, Senior Minister of State, Ministry of Digital Development and Information & Ministry of Health. The Certified Educator in Healthcare Simulation (CEHS) and Certified T
- 김갑성 기자
- 2025-11-06 10:13
-
Ispire Technology Inc.、2026年度第1四半期決算電話会議の開催を予定
ロサンゼルス, 2025年11月6日 -- 電子タバコ技術と精密投与の先駆者であるIspire Technology Inc.(以下「Ispire」または「同社」)(NASDAQ:ISPR)は本日、2025年9月30日に終了した第1四半期の決算結果について説明するため、2025年11月6日(木曜)午前8時(米国東部時間)より決算電話会議を開催することを発表しました。 決算電話会議を聞くには、下記の番号でダイヤル・インしてください。ダイヤル・イン時に案内があった場合は、「Ispire Technology Call」とお申し出ください。 開催日:2025年11月6日(木曜) 時間:午前8時 中央ヨーロッパ時間(ET) ダイヤル・イン番号:米国内からは 844-826-3033、海外からは +1-412-317-5185 におかけください。 この電話会議はウェブキャストでライブ配信され、関係者全員が以下からアクセスできます:https://viavid.webcasts.com/starthere.jsp?ei=1738889&tp_key=82f919c8ba。 通話開始の少なくとも15分前までにリンクにアクセスして登録の上、必要なオーディオ・ソフトウェアをダウンロード、インストールしてください。&n
- 김갑성 기자
- 2025-11-06 09:54
-

The World's First Gene-Editing Therapy Targeting APOC3 for Hy…
[ 메디채널 김갑성 기자 ] CorrectSequence Therapeutics' CS-121 Completed Dosing of First Chylomicronemia Patient, Demonstrating Excellent Safety and Significant Efficacy SHANGHAI, Nov. 6, 2025 -- On November 6, 2025, Shanghai, China, CorrectSequence Therapeutics Co., Ltd. (Correctseq), a clinical-stage biotechnology company pioneering transformer Base Editing (tBE) technology for the treatment of severe diseases, announced that the first patient in its Investigator-Initiated Trial (IIT) of the base-editing therapy CS-121 targeting APOC3 for chylomicronemia / hypertriglyceridemia has successful
- 김갑성 기자
- 2025-11-06 08:00
-

Natural Field will Launch "Ashwagandha White Paper" at FTA F…
HANGZHOU, China, Nov. 5, 2025 -- Natural Field, a leading Chinese supplier of botanical and functional ingredients, is set to release its highly anticipated Ashwagandha White Paper at the prestigious Food Technology and Application (FTA) Forum in Hangzhou. The forum, recognized as one of China's foremost platforms for nutrition and health innovation, brings together leading researchers, industry professionals, and enterprise executives to explore cutting-edge technological advances and market trends. Mr. Haiying Yang, founder of Natural Field, will deliver a keynote address highlightin
- 김갑성 기자
- 2025-11-05 23:00
-
Andor Health's Agentic AI Platform, ThinkAndor®, Receives Fro…
[ 메디채널 김갑성 기자 ] Recognized for its transformational innovation and customer impact in driving the evolution of acute care virtual health through advanced, agentic AI-powered collaboration solutions. SAN ANTONIO, Nov. 5, 2025 -- Frost & Sullivan is pleased to announce that Andor Health has received the 2025 North American Transformational Innovation Leadership Recognition in the acute care virtual health industry for its outstanding achievements in transformational innovation and customer impact. This recognition highlights Andor Health's consistent leadership in driving measurabl
- 김갑성 기자
- 2025-11-05 22:30
- 1포리프 굿마팻 (누바디 굿바이 마이팻) 다이어트 건강기능식품 출시
- 22021년 식품분석전문가 1급/2급 검정자격시험 안내
- 3투썸플레이스, 초콜릿 애프리콧 무스 출시
- 4아임웹, CRM 기능 출시… 고객 행동 데이터 기반 마케팅 지원
- 5하만카돈, AURA STUDIO 4 블루투스 스피커 출시
- 6대구오페라하우스 - 20주년 기념 골든 보이스 시리즈Ⅲ, 테너 콘서트
- 7만성질환자들의 유료독감백신접종에 정책적인 배려가 필요하다
- 82021년 대학생기자단 모집
- 9대한약물영양의학회 2020년 추계학술대회
- 10대한영양제처방학회 춘계학술대회 성황리에 마쳐
2026-01-31_SAT
- 도코모, 아두나와 글로벌 네트워크 API 확장을 위한 파트너십 계약 체결 23:19
- JOHNNIE WALKER TOASTS MUSIC'S BIGGEST WEEKEND WITH THE GO GO HIGHBALL 20:58
- Sanyou Bio's Decade: From an Intelligent Super-Trillion Antibody Library to the "Innovation Hub for Original Drug Discovery" 16:15
- CANADA BACKS NEW DEFENCE, SECURITY AND RESILIENCE BANK 12:05
- Origin Agritech Announces Fiscal Year 2025 Results 06:00
- White Pearl Acquisition Corp. Announces Pricing of $100 Million Initial Public Offering 05:47
- Planet Green Holdings Corp. Provides Response to Unusual Market Action 05:05







