SUZHOU, China, Aug. 26, 2025 -- Recently, Suzhou Basecare Medical Co., Ltd. achieved a major breakthrough—its globally leading Gems culture medium (embryo handling solution VitBase) has officially received registration approval from the National Medical Products Administration (NMPA). This marks not only a milestone event in China's assisted reproductive field but also signifies that domestically produced high-end culture media have officially entered the top international tier, completely breaking the monopoly of imported brands that has lasted for over 30 years.
VitBase belongs to the star product series GEMS developed by Basecare's international brand BMX (formerly Genea Biomedx, a globally renowned IVF center in Sydney). This approval represents a historic leap, elevating Basecare Medical to become one of the very few leading companies in assisted reproductive fluids to simultaneously obtain four major authoritative certifications—CE, FDA, TGA, and NMPA—fully realizing a strategic layout from international acquisition to local transformation, and from R&D to commercialization.
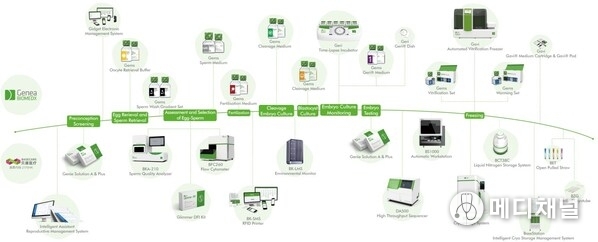
For a long time, China's embryo culture medium market has been highly monopolized by international manufacturers, creating significant uncertainty in the supply chain. The emergence of Basecare's Gems culture medium has completely reshaped the industry landscape. This product incorporates over 30 years of gold-standard clinical practice data from the Sydney IVF Center. Through localized intelligent manufacturing and stringent quality control, its quality rivals top international standards while achieving unprecedented improvements in supply stability and accessibility. Currently, 11 products in the series have received EU CE certification, 8 have been approved by the U.S. FDA, and 11 comply with Australian TGA standards, covering the entire assisted reproductive process. This provides a reliable and trustworthy choice for Chinese embryologists and patients.
It is worth emphasizing that the Gems culture medium had previously gained widespread recognition in China through Cook Medical's local registration and promotion, earning the reputation as the "gold standard for embryo culture." Basecare's newly approved VitBase not only represents the first major achievement in the localization of the full GEMS series of 11 products but also signifies the formal rooting of globally leading culture medium technology in China.
This breakthrough will bring three major transformative impacts to China's assisted reproductive industry: first, completely eliminating import dependency and achieving independent and controllable supply chains; second, significantly reducing treatment costs, allowing more families to benefit from accessible reproductive medical services; and third, leading the upgrade of the entire industry chain, building a complete domestic ecosystem from equipment and consumables to culture media.
A representative of Basecare Medical stated: "We are not just launching a product—we are reshaping the new standard for embryo culture in China. The localization of Gems will greatly enhance the success rate of IVF, making the conception of every new life safer and more stable. In the future, Basecare will continue to lead the wave of domestic substitution and promote high-end reproductive medical products made in China to the world."
Currently, national policies are strongly supporting the development of the assisted reproductive field. The approval of Basecare Medical's Gems culture medium at this pivotal moment not only sets a new benchmark for domestic substitution but also demonstrates the increasingly strong competitiveness and influence of Chinese companies in this field globally. The market expects that this product will soon trigger a new wave of procurement and cooperation, leading the domestic reproductive medical industry into a phase of accelerated development.

