-

The largest pharma show in Asia makes its return to Shanghai this June 2026
SHANGHAI, March 2, 2026 -- CPHI & PMEC China 2026 is set to take place from 16 to 18 June 2026 at the Shanghai New International Expo Centre (SNIEC), building on its extremely successful run over the last few years. As the world's premier destination for pharmaceutical ingredients and manufacturing solutions, this year's edition is set to bring together over 110,000 attendees, and more than 3,600 local and international exhibitors. This comfortably makes CPHI & PMEC China Asia's largest pharma event, offering unparalleled opportunities to source, supply and innovate wit
- 김갑성 기자
- 2026-03-02 20:09
-
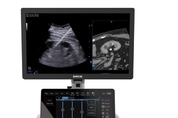
ECR 2026: Esaote Group Introduces Breakthroughs in Ultrasound, MRI and Enterprise Imaging
The new MyLab™E85 GTS and MyLab™C30 GTS Edition ultrasound systems will be presented on Thursday, 5th March at 11.30 a.m. at the Esaote Booth GENOA, Italy, March 2, 2026 -- Esaote Group, a leading Italian innovator in medical imaging – ultrasound, dedicated magnetic resonance and medical IT – attends European Congress of Radiology 2026 in Vienna (March 4-8, Booth 505 Expo Hall X5) with a renewed commitment to supporting radiologists in delivering accurate diagnoses and improved patient outcomes. At ECR 2026, Esaote places particular emphasis on ultrasound innovat
- 김갑성 기자
- 2026-03-02 19:30
-
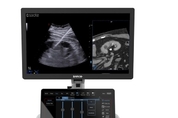
ECR 2026: 에사오테 그룹, 초음파, MRI, 엔터프라이즈 이미징 분야 혁신 기술 공개
신형 MyLab™E85 GTS 및 MyLab™C30 GTS Edition 초음파 시스템, 3월 5일 목요일 오전 11시 30분 에사오테 부스에서 공개 제노바, 이탈리아, 2026년 3월 2일 -- 초음파, 전용 자기공명영상(MRI) 및 의료 IT 등 의료영상 분야를 선도하는 이탈리아 혁신 기업 에사오테 그룹(Esaote Group)이 방사선 전문의들의 더욱 정확한 진단과 향상된 환자 치료 성과 달성을 지원하겠다는 의지를 재확인하기 위해 3월 4일부터 8일까지 오스트리아 빈에서 열리는 2026 유럽방사성학회(European Congress of Radiology 2026, 엑스포 홀 X5 505번 부스)에 참가한다. ECR 2026에서 에사오테는 특히 중재적 방사선학 분야의 초음파 혁신에 중점을 두며, 정밀성과 신뢰성이 필수적인 초음파 유도 시술 및 치료(ultrasound-guided procedures and therapies, GTS) 분야에 대한 헌신을 강화한다. 이러한 헌신은 3월 5일 목요일 오전 11시 30분 에사오테 부스에서 공식 출시되는 신형 MyLab™E85 및 MyLab™C30 GTS
- 김갑성 기자
- 2026-03-02 19:30
-

Shangpu Group Issues Market Position Statement Recognizing SALIAI's Patented Cellular Essence Global Pioneer
BEIJING and GUANGZHOU, China, March 2, 2026 -- SALIAI ("Guangzhou SALIAI Stem Cell Science and Technology Co., Ltd." and "Guangzhou SALIAI Biological Gene Engineering Co., Ltd.," collectively referred to as "SALIAI") is a China-based biotechnology company engaged in stem cell research and related commercial applications. The Company states that it was the first stem cell-focused company quoted on the National Equities Exchange and Quotations (NEEQ), China's New Third Board market. SALIAI develops and commercializes cellular extract–based products a
- 김갑성 기자
- 2026-03-02 16:14
-

COLUMBIA ASIA HOSPITAL BUKIT JALIL LAUNCHES NEW CATH LAB TO STRENGTHEN HEART CARE SERVICES
The new cardiac catheterisation facility enables faster, minimally invasive treatment and improved access to specialist care, closer to home. KUALA LUMPUR, Malaysia, March 2, 2026 -- Columbia Asia Hospital Bukit Jalil has officially launched its new Cardiac Catheterisation Laboratory (Cath Lab), marking a significant step forward in expanding access to advanced heart and vascular care. The launch was officiated by Columbia Asia Hospital Bukit Jalil Chief Executive Officer Dr Sharonpal Singh together with Consultant Cardiologist Dr Prem Nathan Arumuganathan along with Medical Co
- 김갑성 기자
- 2026-03-02 14:24
-

WuXi Biologics Included in S&P Global Sustainability Yearbook for Fourth Consecutive Year
SHANGHAI, March 2, 2026 -- WuXi Biologics (2269.HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO), announced it has been included in the S&P Global Sustainability Yearbook 2026 ("Yearbook") in recognition of its strong performance in the 2025 Corporate Sustainability Assessment (CSA). The company was ranked top in its industry and achieved a Top 1% S&P Global CSA Score, marking the fourth consecutive year that WuXi Biologics has received this highest level of distinction. The Yearbook aims to highlight individual compa
- 김갑성 기자
- 2026-03-02 11:21
-

AWM Spring Dinner 2026 Gathers Over 70 Insurers to Mark a Year of Corporate Solutions
HONG KONG, March 2, 2026 -- AutoMate Wealth Management Limited (AWM), a leading insurance brokerage firm in Hong Kong, successfully hosted its annual AWM Spring Dinner 2026 on February 27, 2026, at Cloud 39, located in The Henderson — one of Hong Kong's premier international landmarks. The event brought together representatives from over 70 leading international and major local insurance companies, along with business partners, to celebrate shared success and strengthen collaboration. AWM has earned market trust through a professional, innovative, and client–centric approach, o
- 김갑성 기자
- 2026-03-02 10:54
-

XPOVIO® Receives Reimbursement Approval in South Korea for a Second Multiple Myeloma Indication
- XPOVIO® is the first XPO1 inhibitor approved for reimbursement by South Korea's National Health Insurance Service (NHIS) for the treatment of adult patients with multiple myeloma (MM). - In South Korea, XPOVIO® has been approved for three indications across MM and diffuse large B-cell lymphoma (DLBCL), and two of these approved indications have been included in the national reimbursement scheme. SHANGHAI and HONG KONG, March 2, 2026 -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK) , a leading innovative, commercial-stage global biotech c
- 김갑성 기자
- 2026-03-02 08:30
-
Lynk Pharmaceuticals Announces Positive Phase III Topline Data of Zemprocitinib in Moderate-to-Severe Atopic Dermatitis
[ 메디채널 김갑성 기자 ] SHANGHAI, HANGZHOU, China and BOSTON, March 2, 2026 -- Lynk Pharmaceuticals Co., Ltd. ("Lynk Pharmaceuticals"), a clinical-stage innovative drug development company focused on therapies for immune and inflammatory diseases, today announced positive topline results from its Phase III clinical trial evaluating zemprocitinib in patients with moderate-to-severe atopic dermatitis (AD). The study met all co-primary and key secondary endpoints, with both dose groups demonstrating highly and statistically significant improvements versus placebo (p < 0.0001), together wi
- 김갑성 기자
- 2026-03-02 08:00
-
Jaypirca® (Pirtobrutinib) Approved in China for the Treatment of Relapsed or Refractory Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma
SAN FRANCISCO and SUZHOU, China, March 2, 2026 -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures, and commercializes high-quality medicines for the treatment of oncologic, autoimmune, cardiovascular and metabolic, ophthalmologic, and other major diseases, announces the non-covalent (reversible) Bruton's tyrosine kinase (BTK) inhibitor, Jaypirca® (pirtobrutinib), has received approval by the National Medical Products Administration (NMPA) in China for a new indication for the treatment of adult patients wit
- 김갑성 기자
- 2026-03-02 08:00
- 1포리프 굿마팻 (누바디 굿바이 마이팻) 다이어트 건강기능식품 출시
- 22021년 식품분석전문가 1급/2급 검정자격시험 안내
- 3투썸플레이스, 초콜릿 애프리콧 무스 출시
- 4하만카돈, AURA STUDIO 4 블루투스 스피커 출시
- 5아임웹, CRM 기능 출시… 고객 행동 데이터 기반 마케팅 지원
- 6대구오페라하우스 - 20주년 기념 골든 보이스 시리즈Ⅲ, 테너 콘서트
- 7만성질환자들의 유료독감백신접종에 정책적인 배려가 필요하다
- 82021년 대학생기자단 모집
- 9대한약물영양의학회 2020년 추계학술대회
- 10대한영양제처방학회 춘계학술대회 성황리에 마쳐
2026-03-02_MON
- The largest pharma show in Asia makes its return to Shanghai this June 2026 20:09
- ECR 2026: Esaote Group Introduces Breakthroughs in Ultrasound, MRI and Enterprise Imaging 19:30
- ECR 2026: 에사오테 그룹, 초음파, MRI, 엔터프라이즈 이미징 분야 혁신 기술 공개 19:30
- Galaxy AI Expands Multi-Agent Ecosystem To Give Users More Choice and Flexibility 18:45
- Samsung Puts AI-Driven Innovation Front and Centre With The Launch Of The Galaxy S26 Series & Galaxy Buds4 Series and the New Galaxy Club 18:42
- TECNO Expands AI Ecosystem at MWC 2026, Forging the "Connection of Intelligence" 17:00
- ATFX Strengthens Strategic Engagements Across Key Financial Hubs 17:00









