-

Yuwell Medical Makes Its Debut at CES 2026: AI Expands the Boundaries of Health Management
LAS VEGAS, Jan. 9, 2026 -- From January 6 to 9, CES 2026, held in Las Vegas—brought together 4,112 leading enterprises from around the world. As a leading Chinese medical device company, Yuwell Medical made its debut at CES. At the exhibition, Yuwell Medical showcased a comprehensive product portfolio covering respiratory health, chronic disease management, first aid, and smart wearables. Respiratory products such as the Pocket S portable breathcare device, SP-6Pro portable oxygen concentrator emphasize mobility in medical use scenarios. Meanwhile, the YE699 fully automatic ele
- 김갑성 기자
- 2026-01-09 12:55
-

Professor Wong Tin Chee Honored as GQ Hong Kong's Local Hero 2025 Giving Back to Society with TCM Wisdom Launches "The Pain Management CEO" Video Series Sharing the Path to Self-Management of Pain Conditions
HONG KONG, Jan. 9, 2026 -- Wong Tin-Chee, Founder of Herbalgy Pharmaceutical Ltd. and Professor of Traditional Chinese Medicine, has been honored as one of the Local Heroes in GQ Hong Kong's Men of the Year 2025, in recognition of his long-standing commitment to advancing pain management through Traditional Chinese Medicine, as well as his dedication to giving back to society through education, philanthropy, and public health advocacy. Download high-resolution images: https://bit.ly/4anXYEU Beyond clinical practice and product development, Professor Wong has cons
- 김갑성 기자
- 2026-01-09 10:00
-

클리노바, 에어플로텍 및 TES-클린 에어 시스템 전략적 인수로 여과 산업 포트폴리오 강화 및 초청정 통제 환경 시장 진출
채터누가, 테네시, 2026년 1월 8일 -- 1월 8일 첨단 산업용 여과 솔루션 분야를 선도하는 글로벌 제조기업 클리노바(Cleanova)가 미국 소재 기업인 에어플로텍(Airflotek)과 TES-클린 에어 시스템(TES-Clean Air Systems)을 전략적으로 인수했다고 발표했다. 1982년 설립된 에어플로텍은 반도체, 제약, 바이오 등 대기질 요건이 까다로운 산업 분야를 대상으로 전 세계 최첨단 클린룸 환경에 사용되는 맞춤형 공기 여과 및 통제 환경 시스템을 설계•제작하고 있다. 1986년 설립된 TES-클린 에어 시스템은 에어플로텍 제품의 독점 유통사로서 수십 년간 축적해 온 클린룸 및 반도체 애플리케이션 전문성을 기반으로 전 세계 고객을 지원한다. 이번 인수로 클리노바는 신뢰성, 성능, 오염 제어가 미션 크리티컬(mission-critical) 요소인 초청정 통제 환경 분야에 즉시 진입하게 됐다. 이 분야는 AI 기반 반도체 제조, 데이터 센터 및 고성능 컴퓨팅 인프라의 신속한 구축, 무균 제약 및 바이오 생산에 필수적이다. 에어플로텍과 TES-클린 에어 시스템은 미션 크리티컬 클린룸 및 초청정 공기
- 김갑성 기자
- 2026-01-08 23:25
-

IMG Opens Applications for 2026 Spring Semester 'Student Journey Scholarship'
INDIANAPOLIS, Jan. 8, 2026 -- IMG (International Medical Group) is now accepting applications for its Student Journey Scholarship, a program designed to help international students fund their studies at a U.S. college or university. Entries for the 2026 Spring semester will be accepted January 1-March 31, 2026, and one student will be selected to receive a $5,000 scholarship. To apply for IMG's Student Journey Scholarship, applicants are asked to submit a 500-word essay or 3-minute video describing their journey that led them to pursue higher education in the United Stat
- 황정호 기자
- 2026-01-08 22:00
-
Illumina and PREMIA partner to expand clinical access to CGP in Asia
TAIPEI, Jan. 8, 2026 -- Illumina Taiwan Biotechnology Co., Ltd., and Precision Medicine Asia, Limited (PREMIA), a leading Asian cancer clinical-genomic screening network, today announced the formation of a strategic partnership that will expand access to comprehensive genomic profiling (CGP) for all eligible oncology patients across the Chang Gung Memorial Hospital (CGMH) system. This partnership also aims to support the creation of an Asia-wide clinical-genomic oncology database to advance precision oncology. "We are committed to ensuring that all cancer patients benefit from the
- 김갑성 기자
- 2026-01-08 21:34
-

Telix to Present Pipeline and Commercial Portfolio Overview at J.P. Morgan Healthcare Conference
MELBOURNE, Australia and INDIANAPOLIS, Jan. 8, 2026 -- Telix Pharmaceuticals Limited (ASX: TLX, NASDAQ: TLX, "Telix") today advises that Dr. Christian Behrenbruch, Managing Director and Group CEO, will be presenting at the 44th Annual J.P. Morgan Healthcare Conference being held in San Francisco, CA (U.S.). Telix will host a presentation on Monday, 12 January at 9:00 am PST (12:00 pm EST / 4:00 am AEDT, 13 January), providing an overview of the Company's pipeline assets alongside its commercial portfolio. The event will be webcast live here and a
- 김갑성 기자
- 2026-01-08 19:00
-

From Quantified Meditation to Bio-Computing, All Here Launches the Bio-Intelligence Initiative
GENEVA, Jan. 8, 2026 -- All Here announces the launch of the Bio-Intelligence Initiative, the world's first research program exploring how deep meditative states of stability and silence of Mind give rise to measurable neural patterns, and how these complex neural signatures may inspire the development of biologically linked, energy-efficient, and adaptive AI systems. As artificial intelligence systems continue to scale through increased computing power and energy consumption, All Here is advancing an alternative research pathway grounded in the principles of complex decision m
- 김갑성 기자
- 2026-01-08 18:48
-
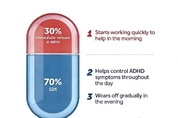
Ark Biopharmaceutical Receives China Marketing Authorization for Aizhida for the Treatment of ADHD
SHANGHAI, Jan. 8, 2026 -- Shanghai Ark Biopharmaceutical Co., Ltd. ("ArkBio") today announced that China's National Medical Products Administration (NMPA) has approved the New Drug Application for Serdexmethylphenidate Chloride and Dexmethylphenidate Hydrochloride Capsules (Aizhida) for treatment of Attention Deficit Hyperactivity Disorder (ADHD) in patients 6 years of age and older in China. Aizhida is a central nervous system stimulant indicated for the treatment of ADHD. Each once-daily capsule contains serdexmethylphenidate (SDX), a prodrug of dexmethylphenidate,
- 김갑성 기자
- 2026-01-08 17:58
-

Cigna Healthcare Hong Kong Launches Critical Illness Plan to Enhance Health and Financial Protection for Customers
Comprehensive coverage for every stage of the health journey, plus a welcome offer supporting mental health HONG KONG, Jan. 8, 2026 -- Cigna Healthcare Hong Kong today announced the launch of the Cigna Healthcare VitalGuard Critical Illness PlanTM (the "Plan"), expanding its medical insurance portfolio with critical illness protection to deliver comprehensive health and financial protection for customers and their families across every stage of life. Jonathan Spiers, CEO at Cigna Healthcare Hong Kong, said: "As healthcare costs rise and criti
- 김갑성 기자
- 2026-01-08 17:13
-

비바시스템즈, 노보 노디스크 국제 사업부에 '비바 볼트 CRM' 공급
[ 메디채널 김갑성 기자 ] 차세대 CRM 기능과 에이전틱 AI로 영업 역량 극대화 서울, 대한민국, 2026년 1월 8일 -- 비바시스템즈(Veeva Systems, NYSE: VEEV)는 오늘 노보 노디스크(Novo Nordisk)의 국제 사업부(International Operations)에서 비바 볼트 CRM(Veeva Vault CRM)) 도입을 확정했다고 발표했다. "노보 노디스크와의 파트너십을 볼트 CRM(Vault CRM)까지 확장하게 되어 영광입니다,"라고 비바 CEO 피터 가스너(Peter Gassner)가 말했다. "AI 기반 기술을 통한 양사의 협력을 바탕으로 노보 노디스크가 중증 만성 질환을 극복할 혁신 신약을 공급하고, 전 세계 환자들에게 장기적인 건강을 선사할 수 있도록 지원하겠습니다." 볼트 CRM은 대면 및 디지털 채널 전반에 걸쳐 보다 효과적인 영업 활동을 이끌어내는 심층적인 애플리케이션과 에이전틱 AI(Agentic AI)를 갖춘 볼트 CRM 스위트의 일부이다. 볼트 CRM 스위트는 고도화된 글로벌 대응 역량을 바탕으로, 제약 업계
- 김갑성 기자
- 2026-01-08 13:19
- 1포리프 굿마팻 (누바디 굿바이 마이팻) 다이어트 건강기능식품 출시
- 22021년 식품분석전문가 1급/2급 검정자격시험 안내
- 3투썸플레이스, 초콜릿 애프리콧 무스 출시
- 4하만카돈, AURA STUDIO 4 블루투스 스피커 출시
- 5아임웹, CRM 기능 출시… 고객 행동 데이터 기반 마케팅 지원
- 6대구오페라하우스 - 20주년 기념 골든 보이스 시리즈Ⅲ, 테너 콘서트
- 7만성질환자들의 유료독감백신접종에 정책적인 배려가 필요하다
- 82021년 대학생기자단 모집
- 9대한약물영양의학회 2020년 추계학술대회
- 10대한영양제처방학회 춘계학술대회 성황리에 마쳐
2026-03-03_TUE
- US BioTek Laboratories Completes Merger With NutriPATH Pathology 02:15
- Skyhawk Announces Australia's Therapeutic Goods Administration Has Determined SKY-0515 for Huntington's Disease Meets Eligibility Criteria for Registration via the Provisional Approval Pathway 01:20
- Innovaccer Receives Frost & Sullivan's 2026 United States New Product Innovation Recognition for Excellence in AI-Driven Patient Access Solutions 00:57
2026-03-02_MON
- NYSE Content Update: Georgia Gov. Kemp + Atlanta Mayor Dickens to Ring Bell Ahead of World Cup 23:21
- TDK opens its fifth regional headquarters in Asia-Pacific with a new business entity in India 23:00
- The Sigourney Award-2026 Now Accepting Applications for Psychoanalytic Innovation 22:49
- XellSmart Receives Fourth Consecutive US FDA & China NMPA IND Clearance for MSA-P iPSC-derived Cell Therapy 22:10










